New research helps pursuit for malaria vaccine
Using the CLS, SickKids scientists have taken an important step forward on the path to finding effective biomedical interventions to halt the spread of malaria.
By Colleen MacPhersonJean Philippe Julien (left) and his collaborator, Laboratory Research Project Coordinator Anthony Semesi (right), performing lab work at SickKids.
Scientists from The Hospital for Sick Children (SickKids) identify structure of key malaria protein
Using technology available at the Canadian Light Source synchrotron, SickKids scientists have taken an important step forward on the path to finding effective biomedical interventions to halt the spread of malaria, a disease that affected an estimated 216 million people worldwide in 2016 alone.
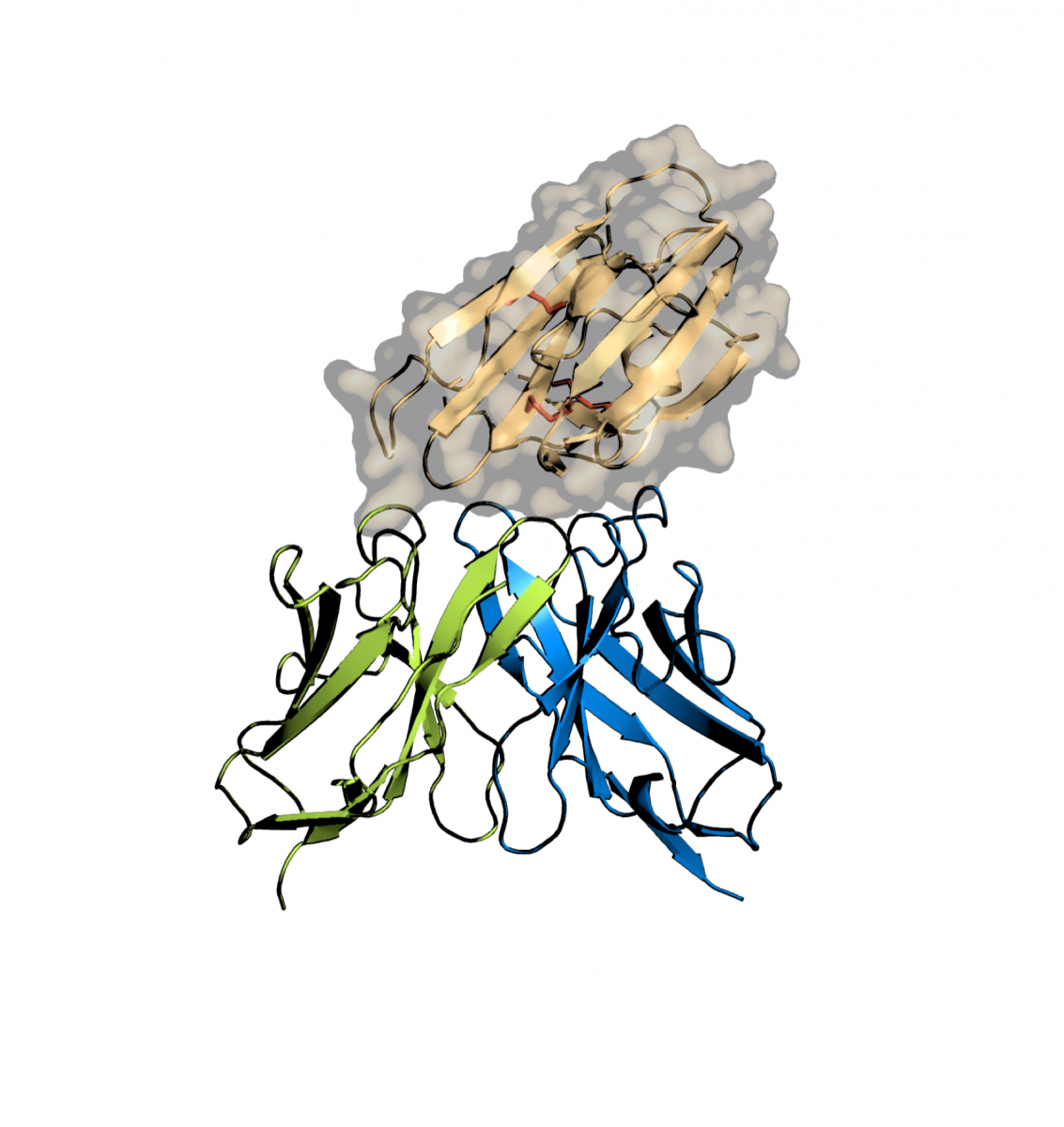
Jean-Philippe Julien, a scientist in the Molecular Medicine program at SickKids, and his colleagues focused on a molecule known to be essential for the malaria parasite Plasmodium falciparum to go through the sexual stages of its lifecycle. Disrupting that stage of the lifecycle has the potential to reduce infections and deaths from malaria because parasite transmission between humans would be blocked by inhibiting parasite development in the Anopheles mosquito.
“The protein we looked at was identified several years ago as an important target for malaria parasite biology,” says Julien, who is also a Canada Research Chair in Structural Immunology and an Assistant Professor in the Departments of Biochemistry and Immunology at the University of Toronto. “The field has tried for over a decade to clarify its structure in order to guide the development of biomedical interventions that can curb the spread of malaria.”
Julien explained that people with malaria can naturally produce antibodies that bind this protein and can interrupt the lifecycle of the parasite. If a mosquito bites someone with malaria who made these types of antibodies, the transmission rate of the disease is subsequently greatly reduced. The key to designing vaccines is to elicit enough of these types of antibodies in the general population. Having an understanding at the molecular level of how these blocking antibodies bind the protein provides clues for vaccine design.

The research done by Julien and his team at the CLS and SickKids showed what the protein looks like, and how such an antibody binds it to block its function. This information can now be used for vaccine development. In addition, the antibody itself can be used as an intervention when administered to people in malaria-endemic areas, an approach that is called passive immunization.
With the molecule design complete, Julien said it is being produced in preparation for clinical trials in a malaria-endemic region, “which will start to test how effective this biomedical intervention can be at reducing malaria transmission.
The research results will advance the researchers’ continuing investigations into vaccine development. In addition, the research team wants these results to be available for anybody to use with new ideas. To that end, the group published its findings in an open-access journal, Nature Communications, and deposited structures related to this study and solved from data collected at CLS in the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank, which is accessible by all researchers around the world.
Kundu, Prasun, Anthony Semesi, Matthijs M. Jore, Merribeth J. Morin, Virginia L. Price, Alice Liang, Jingxing Li et al. "Structural delineation of potent transmission-blocking epitope I on malaria antigen Pfs48/45." Nature communications 9, no. 1 (2018): 4458. DOI: 10.1038/s41467-018-06742-9
To arrange an interview, contact:
Victoria Martinez
Communications Coordinator
Canadian Light Source
306-657-3771
victoria.martinez@lightsource.ca
