X-rays capture ageing process in EV batteries
Canadian researchers capture x-ray images of electric vehicle batteries as they degrade over time
By Victoria Martinez
CLS researcher Toby Bond uses x-rays to help engineer powerful electric vehicle batteries with longer lifetimes. His research, published in The Journal of the Electrochemical Society, shows how the charge/discharge cycles of batteries cause physical damage eventually leading to reduced energy storage. This new work points to a link between cracks that form in the battery material and depletion of vital liquids that carry charge.
Bond uses the BMIT facility at the Canadian Light Source at the University of Saskatchewan to produce detailed CT scans of the inside of batteries. Working with Dr. Jeff Dahn at Dalhousie University, he specializes in batteries for electric vehicles, where the research imperative is to pack in as much energy as possible into a lightweight device.
“A big drawback to packing in more energy is that generally, the more energy you pack in, the faster the battery will degrade,” says Bond.
In lithium-ion batteries, this is because charging physically forces lithium ions between other atoms in the electrode material, pushing them apart. Adding more charge causes more growth in the materials, which shrink back down when the lithium ions leave. Over many cycles of this growing and shrinking, micro-cracks begin to form in the material, slowly reducing its ability to hold a charge.
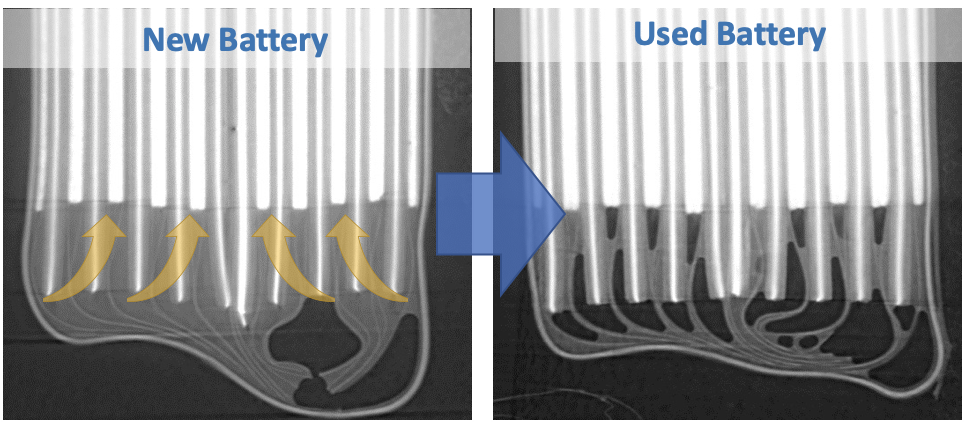
“It can eventually cause the materials in the battery to crumble from the inside out. If it gets bad enough, it can cause parts of the battery to actually peel off inside itself,” says Bond. “And if it causes large-scale damage inside the battery, that can become a safety issue as well.”
Studying this problem, and how effective coatings and other treatments are in stopping it, has been important in the field for a long time. Traditionally, the cracks forming in a battery have been studied by taking the battery apart and looking at individual particles under an electron microscope. This destroys the battery, so it doesn’t allow researchers to preserve the larger structure and see what other effects this cracking might have on the rest of the battery.
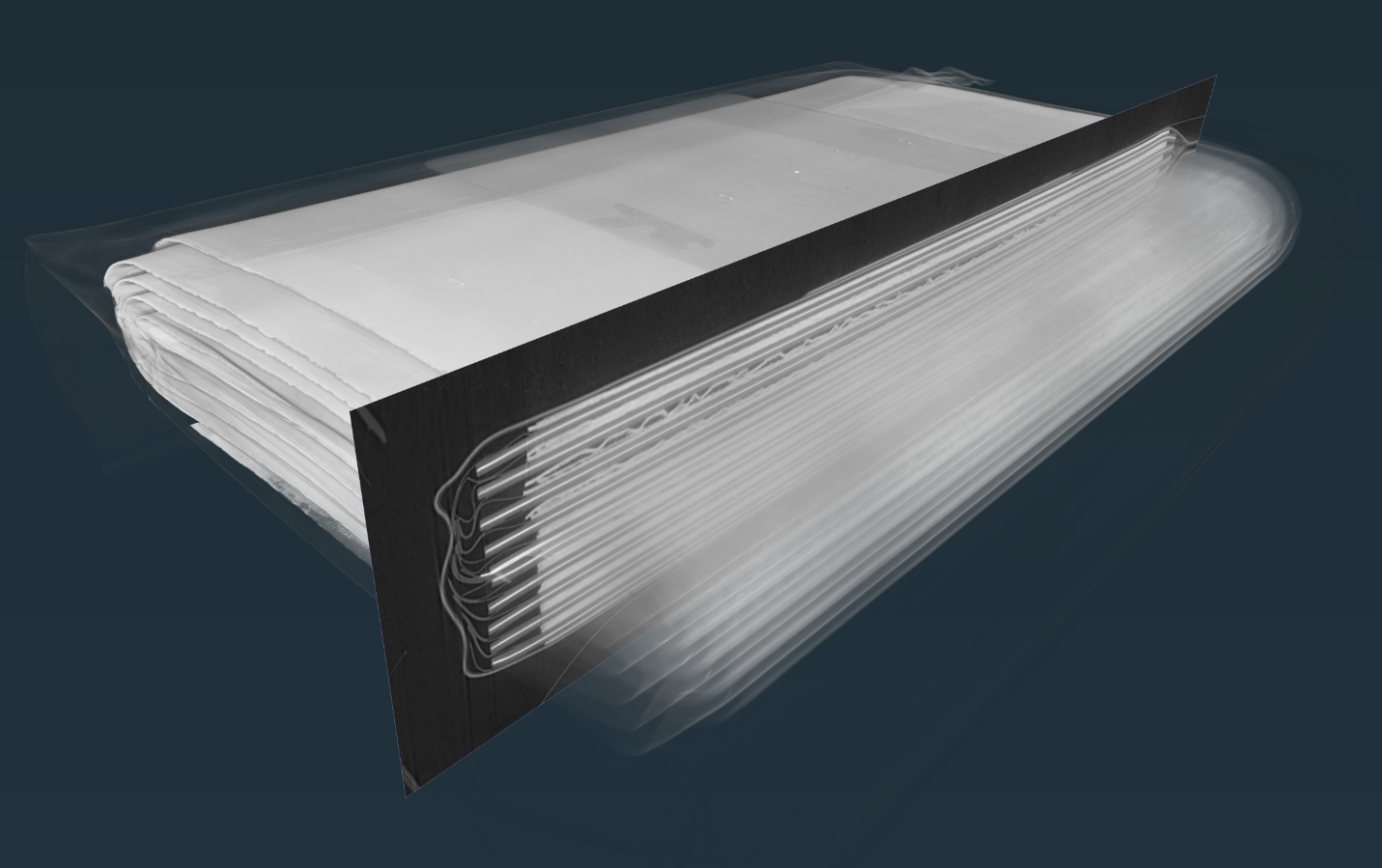
By using x-ray imaging at the CLS, Bond says researchers can study these effects in context, and see how cracking causes changes in the rest of the battery. In this study, the researchers discovered that as micro-cracking in the battery got worse, liquids in the cell were sucked up into the extra space between the cracks, which may not leave enough liquid to go around.
“This is the first time anyone's been able to capture all of these effects happening together in a working battery,” says Bond. “This depletion of liquid electrolyte can cause serious problems, since any part of the battery that doesn’t get enough liquid would essentially stop working.”
In this study, Bond and colleagues studied batteries that had been continuously charged and discharged to different levels over years, alongside otherwise-identical batteries that had not been used at all. The 3D x-ray scans they collected using BMIT’s bright, focused light allowed them to see precisely how different materials were affected by use, both at the microscopic scale and throughout the entire battery.
A practical takeaway? The team found that draining the battery a small amount caused less deterioration than discharging the battery all the way. This is likely because a smaller change in charge causes less physical strain on the battery electrode materials over time. This effect is important to understand for new applications such as long-haul transport, electric aircraft, and using parked electric vehicles to store and deliver energy into the electrical grid. These scenarios often require using more of the battery’s full capacity before being recharged.
“As we start replacing more and more combustion-driven vehicles with electric vehicles, it’s really important to understand how batteries will behave under different conditions,” Says Bond. “It’s very exciting to work on these problems, and we really need tools like synchrotrons to understand the fine details of what’s going on inside the battery when we try out new approaches.”
Photos: Synchrotron | BMIT beamline at CLS (used by the researchers) | Story Photos
Media Relations:
Victoria Martinez
Communications Coordinator
Canadian Light Source
306-657-3771
victoria.martinez@lightsource.ca
