The future of stroke treatment
A team of international collaborators has been researching a promising new therapeutic for the treatment of strokes and other brain injuries.
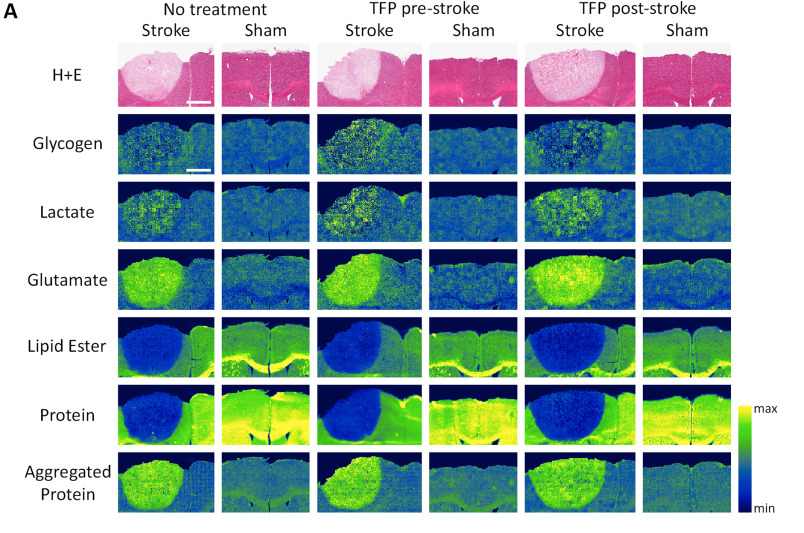
By Erin MatthewsRepresentative images of FTIR spectroscopic imaging.
In a recent paper published in BBA-Biomembranes, researchers from the University of Saskatchewan, Columbia University, and the University of Oxford, have used synchrotron imaging, combined with traditional techniques, to assess the effects of an FDA-approved antipsychotic medication, known as trifluoperazine (TFP), during the acute phase post stroke.
With the help of the Canadian Light Source (CLS) at the University of Saskatchewan (USask) and the Stanford Synchrotron Radiation Light Source (SSRL), the team was able to measure subtle changes in the biochemistry of brain tissue – measurements that are only possible with the use of a synchrotron.

“The synchrotron at the CLS provides us with a tool that allowed us to map unprecedented levels of brain detail,” said Dr. Mootaz Salman, Research Scientist in the Department of Physiology, Anatomy and Genetics at Oxford and Junior Research Fellow at Wolfson College.
Already approved for human use in the treatment of schizophrenia, the team believes that TFP has the potential to stop the swelling in the brain that occurs after a stroke or other cerebral injury.
“According to the World Health Organization, around 60 million people sustained a traumatic brain or spinal cord injury and a further 15 million people suffered from a stroke in 2020,” said Salman. “Edema, which is swelling due to water or other body fluid accumulation, is the hallmark of stroke and plays a major role in stroke-associated morbidity and mortality.”
USask professor and Saskatchewan Clinical Stroke Research Chair Dr. Michael Kelly, says that the CLS played an important role in understanding how TFP could be used for stroke patients. “Synchroton imaging has facilitated research on effects of stroke on brain energy metabolism and elemental distribution, which has given us new insights into stroke treatment.”
Salman and Kelly’s team has demonstrated that a dose of TFP reduces edema in a mouse model of stroke—a breakthrough that could lead to the development of new treatment options for stroke patients.
TFP acts on doughnut-shaped water channel proteins, called aquaporins, in brain cells. During a stroke, the brain’s blood supply is restricted, which prevents cells from receiving enough oxygen. These oxygen-starved cells are unable to do their usual job of maintaining a balance of fluid, nutrients and electrolytes within the brain, leading to severe swelling.
“Water rushes from the outside through these doughnut-shaped proteins, into the cells that then swell,” Dr. Salman said. “The build-up of pressure damages the fragile brain tissue, disturbing the flow of electrical signals from the brain to the body.”
TFP stops this from happening by preventing a signal that would normally cause more aquaporin channels to rise to the surface of brain cells. Other treatments — which often include invasive surgeries — are techniques used to manage symptoms and minimize damage that has already occurred.
TFP is already a licensed medicine, Salman says it could be rapidly repurposed for use in new therapeutic protocols for stroke during the early acute phase.
“Our novel approach offers new hope for patients with central nervous system injuries and strokes and has a huge therapeutic potential. These findings suggest it could be a good candidate for early phase of human clinical application at a low treatment cost in the near future,” Dr. Salman said.
Sylvain, Nicole J., Mootaz M. Salman, M. Jake Pushie, Huishu Hou, Vedashree Meher, Rasmus Herlo, Lissa Peeling, and Michael E. Kelly. "The effects of trifluoperazine on brain edema, aquaporin-4 expression and metabolic markers during the acute phase of stroke using photothrombotic mouse model." Biochimica et Biophysica Acta (BBA)-Biomembranes 1863, no. 5 (2021): 183573. DOI: 10.1016/j.bbamem.2021.183573
For interviews, please contact:
Victoria Schramm
Communications Coordinator
Canadian Light Source
306-657-3516
victoria.schramm@lightsource.ca
