Finding the right puzzle piece to stop cancer growth
McGill researchers use the CLS to analyze a new class of compounds that could help develop novel therapies for age-related cancers.
By Sarath PeirisDr. Youla Tsantrizos (left) with student Yuting Feng.
A research team from McGill University has used the Canadian Light Source (CLS) at the University of Saskatchewan to study a new class of compounds that could help develop novel therapies for age-related cancers, including prostate and breast cancer. Cancers are second only to heart disease as the leading cause of death in people over age 60.
“As we age, it is very clear that our biochemistry starts to be modified. Additionally, cancer cells take over our metabolic pathways and the nutrients in our body in order to fuel their own accelerated growth,” said researcher Youla Tsantrizos, a professor in McGill’s chemistry and biochemistry departments.
In a paper published in the Journal of Medicinal Chemistry, her team details how they are developing novel compounds to control the spread of cancer.
“We used X-ray crystallography at the CLS to see, at the molecular level, the interactions between potential human therapeutics and their biological target,” she said.
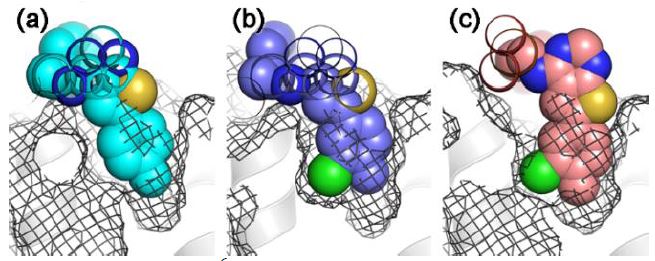
The researchers noticed something highly unusual: “Molecules of compounds that had the same structure but are mirror images of each other, like two hands, were both binding to the target enzyme and inhibiting it,” Tsantrizos said. However, they were binding in different orientations that corresponded with their shape (or chirality).
Tsantrizos said CLS crystallography was integral in showing that molecular compounds with the same chirality, even though they had different structures, all bound to the target enzyme in the same orientation, while those with the opposite shape (chirality) bound in the opposite orientation.
By determining which form of molecules help to stop cancer growth and how, researchers can now develop more effective inhibitors that have a good chance of becoming cancer therapeutics, she said.
“What we were able to show in this paper is that the chirality of molecules controls their interactions with the biological target we are trying to inhibit. That knowledge is critical for allowing us to optimize compounds into better therapeutics for treating cancer,” she said.
Tsantrizos said the researchers are excited about their findings, although bringing an actual drug to market is a long and expensive process.
“Our hope is that we can bring our project into an advanced enough stage in the research process so that a large pharmaceutical company would be interested in collaborating with us to bring a drug to clinical development.”
Tsantrizos said that drug discovery is a “team sport” and requires diverse research expertise in order to be successful. She expressed her gratitude to her expert crystallography colleagues Professor Albert Berghuis and Dr. Jaeok Park (now a professor at Memorial University) and Matthew A. Schilling, as well as her students, Yuting Feng, Rebecca Boutin, Peter Viereck, and Post-Doctoral Fellow, Dr. Shi-Guang Li.
For more information, contact:
Victoria Schramm
Communications Coordinator
Canadian Light Source
306-657-3516
victoria.schramm@lightsource.ca
