Diabetes discovery challenges known research
A discovery by an international group of scientists challenges known research on diabetes and may open the door to new therapeutic approaches for the disease that affects nearly 500 million people globally.
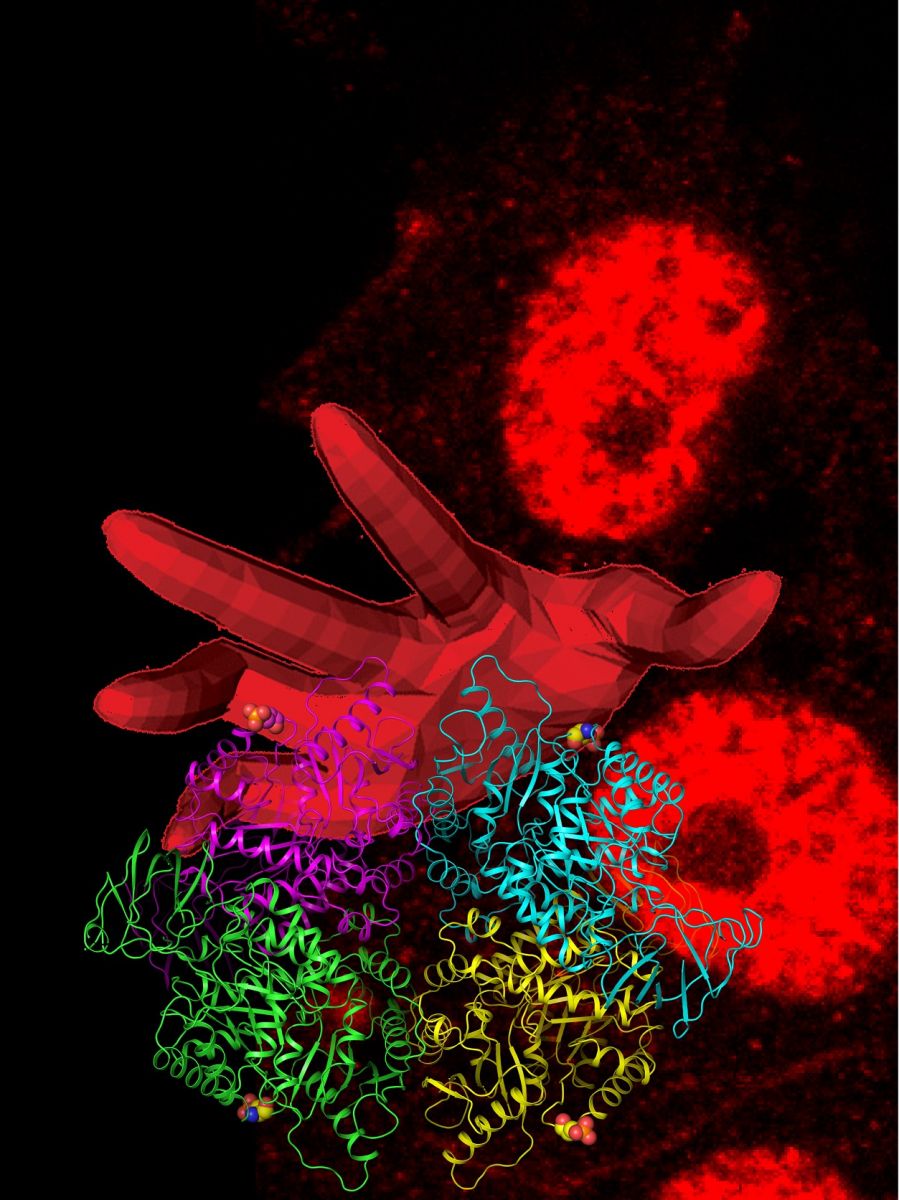
By Sarath PeirisDepiction of the “hand” of CDK reaching out to sequester PKL in the hepatocyte nucleus. Art by J. Rinehart and B. Gassaway.
Yale University scientists and colleagues who used the CLS share findings that could lead to a new therapeutic approach to treating diabetes.
A discovery by an international group of scientists challenges known research on diabetes and may open the door to new therapeutic approaches for the disease that affects nearly 500 million people globally.
Their research focused on pyruvate kinase, an enzyme that is involved in communication at the cell level through a process known as protein phosphorylation, which changes the shape of a protein and alters how that protein behaves.
The study is a piece of a larger project that has researchers looking at how different signals, like insulin levels, are interpreted in the liver.
“We set out to understand and characterize insulin signalling in a laboratory model, and we found some activities in that model that were contrary to the textbooks,” said Jesse Rinehart, associate professor in the Department of Cellular & Molecular Physiology at the Yale University School of Medicine.
The team’s findings were published in Cell Reports and have opened up a new area of insight and exploration in an already highly active field of research.
“While we understand fundamentally that protein phosphorylation is key to how cells, organs and organisms like humans process signals and relay information, there is still a tremendous amount of information we don’t understand,” Rinehart said.
The team is still trying to find out what happens to a cell directly when a protein is phosphorylated.
The current understanding is that phosphorylation inactivates pyruvate kinase. The enzyme is a key factor in metabolism, which extracts energy from food. Alterations in the activity of pyruvate kinase is thought to play a role in diabetes.

The researchers were surprised when their results differed from what is currently known about signaling pathways involving pyruvate kinase.
“If phosphorylation turns off the enzyme, then in that experiment we should be able to have an enzyme that is active and one that is inactive,” Rinehart said. “When we made those enzymes there was no difference. So, what we thought was a textbook off-switch turned out to do nothing to the enzyme activity.”
The next step was to solve the structure of the proteins and determine enzyme activity using the CMCF beamline at the Canadian Light Source at the University of Saskatchewan.
Rinehart and his team were able to confirm that the enzyme as still active despite being phosphorylated. The structures showed that the phosphorylated proteins were also no different from the non-phosphorylated proteins and thus confirmed the activity measurements.
The team then used cell biology techniques in liver cells to show that the new pyruvate kinase phosphorylation site dictates where the protein is localized within the cell. Their worked showed the phosphorylated enzyme is targeted to the nucleus a site typically associated with DNA storage and gene expression in the cell.
The study opens up new questions about the enzyme and how it responds to signals from insulin.
Rinehart stressed that the team has not discovered a new way to treat diabetes but have helped contribute to our foundational understanding of the disease.
“This really inspires us to keep digging into problems in these areas that are well studied but also very challenging. We hope to find new insights, and hope that our contribution to the field really can direct or open up a new avenue to think about a new therapeutic approach,” Rinehart said.
Research team members were from Yale University and Veterans Affairs Medical Centre in Connecticut, Agios Pharmaceuticals in Massachusetts, and Viva Biotech in Shanghai, China.
Gassaway, Brandon M., Rebecca L. Cardone, Anil K. Padyana, Max C. Petersen, Evan T. Judd, Sebastian Hayes, Shuilong Tong et al. "Distinct Hepatic PKA and CDK Signaling Pathways Control Activity-Independent Pyruvate Kinase Phosphorylation and Hepatic Glucose Production." Cell reports 29, no. 11 (2019): 3394-3404. https://www.sciencedirect.com/science/article/pii/S2211124719314809.
For more information, contact:
Victoria Schramm
Communications Coordinator
Canadian Light Source
306-657-3516
victoria.schramm@lightsource.ca
