New catalyst could be key to a sustainable future
Scientists develop new, greener technique to produce hydrogen peroxide, used in mining, textiles, and cosmetics industries.
By Victoria MartinezSunbeams peek through a cloud over a mountainous landscape.
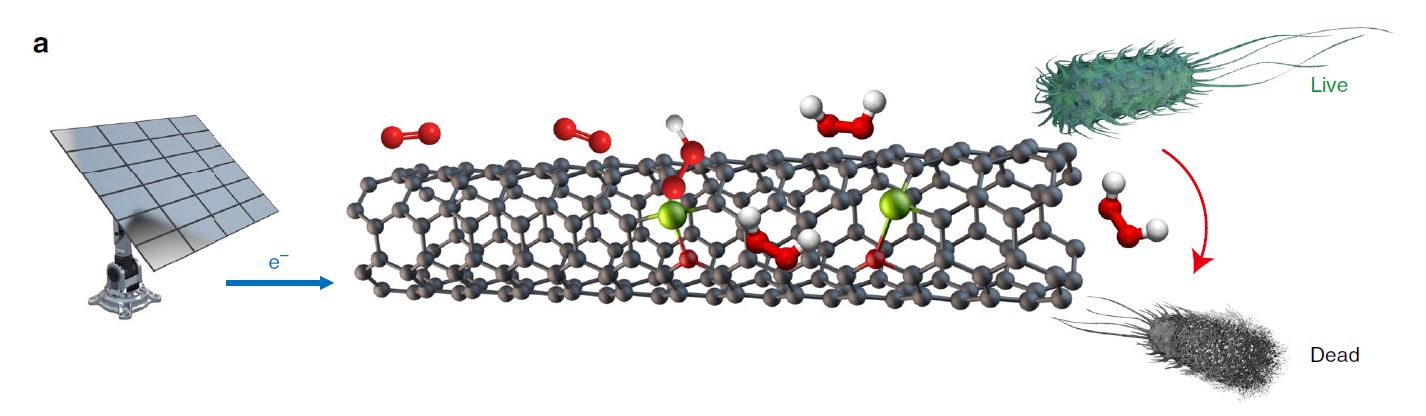
A multi-institution team led by Harvard University, Stanford University, Rice University and University of Calgary scientists has developed a new, greener technique to produce hydrogen peroxide. The technique uses an electrochemical reaction to produce the chemical, and the catalyst they identified is the most efficient currently known to exist.
The results were recently published in Nature Communications.
Hydrogen peroxide, best known as a mild antiseptic available at drugstores or for its applications in lightening hair, is also regularly used as a bleaching agent in textiles, cosmetics, and food services. Many other industries, like mining, use it as an oxidizing agent.
"The current industrial method for producing hydrogen peroxide is pretty energy-intensive," says Dr. Seoin Back, one of the first authors of the paper. He was a postdoctoral researcher at Stanford University during this project and now works at Carnegie Mellon University in Pennsylvania. "Electrochemical production of hydrogen peroxide is one possible solution to overcome the weaknesses of the current method."
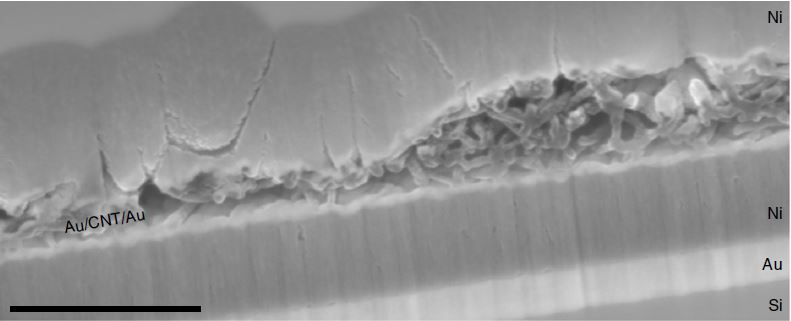
The project used the Canadian Light Source at the University of Saskatchewan to get bulk information about the electrochemical reaction and the atomic structure in the catalysts, which was complemented with theoretical work and scanning transmission electron microscopy.
Dr. Kun Jiang, who was a postdoctoral researcher at Harvard during this project, describes the synchrotron tools as “state-of-the-art” and key to gathering information on single-atom catalysts like the one studied in this project.
The electrochemical method uses a catalyst to convert molecular oxygen (the O2 molecule) into hydrogen peroxide. Molecular oxygen can also be converted into water using the technique; research into the best way to produce hydrogen peroxide is a newer research area.
The carbon-iron-oxygen catalytic site identified in their work is the most efficient identified to date.
"Catalysis is a key for a sustainable future - we'll be able to produce renewable electricity cheaply for electrochemical processes and replace more temperature- and pressure-based production techniques for many daily basis chemicals," says Back.
Another advantage of this production technique is its flexibility and small scale.
"This electrochemical process is also much smaller than the current temperature- and pressure-based method, so you can eliminate the transportation of potentially dangerous hydrogen peroxide. Rather, we can produce hydrogen peroxide when needed," says Back.
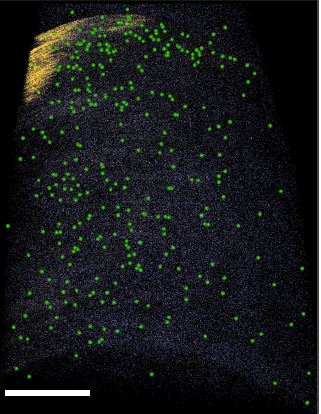
One particular application for this electrochemical process is for water treatment, where the team showed rapid disinfection efficiency for E-coli bacteria (43% bacteria inactivation in 5 minutes of operation and 100% inactivation after a two hour operation).
While this project focused on hydrogen peroxide, Jiang is excited by the potential this technique has for other applications.
"We can fine-tune different structures to target different products for many different applications," says Jiang, who continues to work on electrochemical production research.
Jiang, Kun, Seoin Back, Austin J. Akey, Chuan Xia, Yongfeng Hu, Wentao Liang, Diane Schaak et al. "Highly selective oxygen reduction to hydrogen peroxide on transition metal single atom coordination." Nature communications 10, no. 1 (2019): 1-11.https://www.nature.com/articles/s41467-019-11992-2
Kun Jiang is an Associate Professor at the Institute of Fuel Cells at Shanghai Jiao Tong University, China. Seoin Back is a postdoctoral researcher with the Department of Chemical Engineering at Carnegie Mellon University in Pennsylvania. He was a postdoctoral researcher at Stanford University during this project. Samira Siahrostami is an Assistant Professor with the Department of Chemistry at the University of Calgary. She was an Associate Staff Scientist at Department of Chemical engineering at Stanford University during this project. Haotian Wang is now a William Marsh Rice Trustee Chair Assistant Professor with the Department of Chemical and Biomolecular Engineering at Rice University. He was a Rowland Fellow, PI, at Harvard University during the project.
For more information, contact:
Victoria Schramm
Communications Coordinator
Canadian Light Source
306-657-3516
victoria.schramm@lightsource.ca
